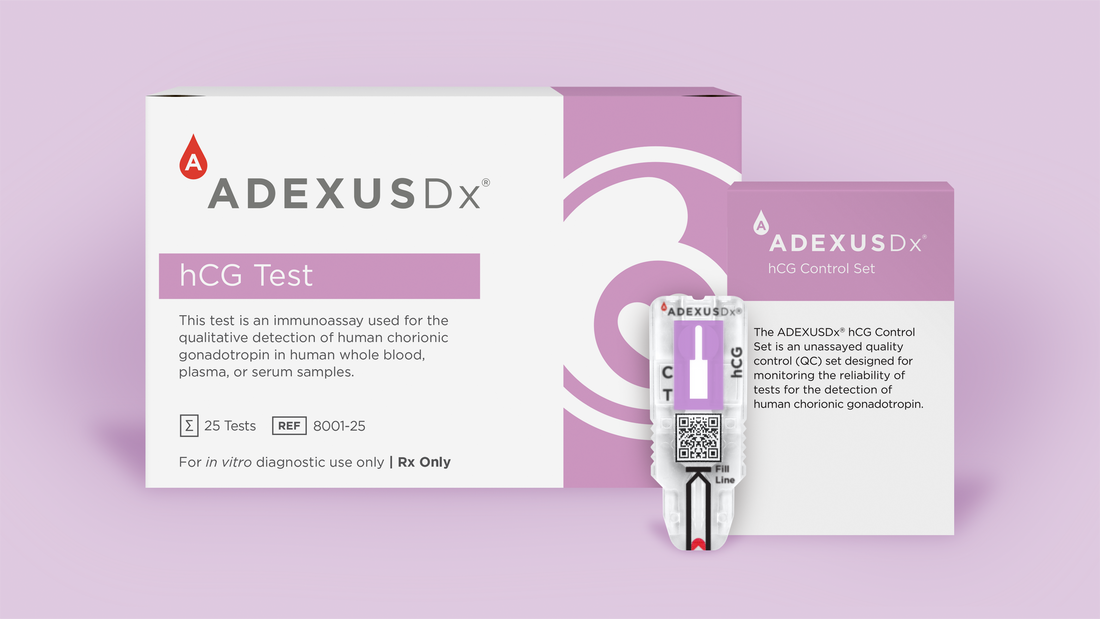
The ADEXUSDx hCG Test, NOWDiagnostics’ first FDA-cleared test available in the United States, is an immunoassay used for the qualitative detection of human chorionic gonadotropin (hCG) in human whole blood, plasma or serum. The test is an early detection aid for pregnancy for health care professionals in a variety of clinical and critical care settings. Depending on the woman and her pregnancy, the ADEXUSDx hCG test can detect pregnancy approximately two to eight days earlier than commonly available urine-based tests. The ADEXUSDx hCG test results are available 10 minutes after the test is used.
Early detection of pregnancy is important for caregivers in order to avoid certain medications and procedures that could harm a fetus. Unfortunately, clinics across the country are experiencing too many false-negative results…read more.