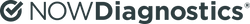Company Receives Funding from Labcorp's Venture Fund to Advance Diagnostic Breakthroughs
SPRINGDALE, Ark., Jan. 9, 2024 /PRNewswire/ -- NOWDiagnostics (NOWDx), a developer of over-the-counter (OTC) and point-of-care (POC) diagnostics tests and first-in-class technology and platforms, today announced they have submitted a De Novo Classification Request to the U.S. Food and Drug Administration (FDA) for the company's First to Know™ at-home syphilis test. Additionally, the company secured a strategic investment from the Labcorp Venture Fund, marking an important milestone in the company's commercialization strategy.
The development of the First to Know™ syphilis test comes as healthcare experts agree there is a syphilis emergency in the U.S., according to the Centers for Disease Control and Prevention (CDC). Once thought to be in decline, syphilis is now resurging at an alarming rate. Left untreated, the consequences can be serious, especially for pregnant women.
"We are excited about the FDA submission as we work to bring this syphilis test to market. This test will meet the consumer's demand for faster, more affordable, and accessible tests in the privacy of their home," said Rob Weigle, CEO of NOWDx. "We are also pleased to have Labcorp as an investor and strategic partner as we begin to launch the first of many OTC tests."
"In support of Labcorp's mission to improve health and improve lives, the Labcorp Venture Fund invests in early stage, private companies like NOWDx that are making healthcare more convenient, accessible, affordable and personalized," said Megann Vaughn Watters, Vice President, New Ventures & Strategic Alliances for Labcorp.
NOWDx is in the process of completing a $15M Series B round led by DigitalDx Ventures. "With the Labcorp investment, along with our current investors and new family offices, the round has closed over $12M to date. We look forward to discussing our vision with the attendees at the J.P. Morgan Healthcare Conference," added Weigle.
"The reality of 'touch to test' technology is a game-changer in the large, critical areas of infectious disease and women's health," said Michele Colucci, founder and managing partner of DigitalDx Ventures, a Silicon-Valley based diagnostics fund focusing on earlier, less invasive, more accurate, and affordable medical tests enabling access to healthcare for all. "The powerful combination of NOWDx's proprietary technology and the ability to produce their tests at scale in their state-of-the-art production facility in the tech hub of Bentonville positions the company to be a market leader meeting the growing consumer need."
Weigle and his executive team are attending the 42nd Annual J.P. Morgan Healthcare Conference in San Francisco on January 8-11, 2024. Please contact (Todd.Grice@nowdx.com) for a meeting time.
About NOWDiagnostics (NOWDx)
NOWDiagnostics develops and manufactures "Touch to Test" over-the-counter and point-of-care diagnostic tests that yield results in minutes. NOWDx's patented approach allows for virtually any immunological assay to be accurately performed onsite in one step using a tiny amount of blood or, in some cases, saliva. We envision a world where people have greater access to information concerning their health and well-being and are more comfortable shaping their desired outcomes. NOWDx is committed to changing healthcare by providing accessible, affordable, and accurate consumer testing worldwide. nowdx.com
NOWDx CONTACT:
Todd Grice
todd.grice@nowdx.com
717.380.9400